There might be some bad news for UK Apple fans when it comes to obtaining one of the amazing new Apple Watch Series 4 devices.
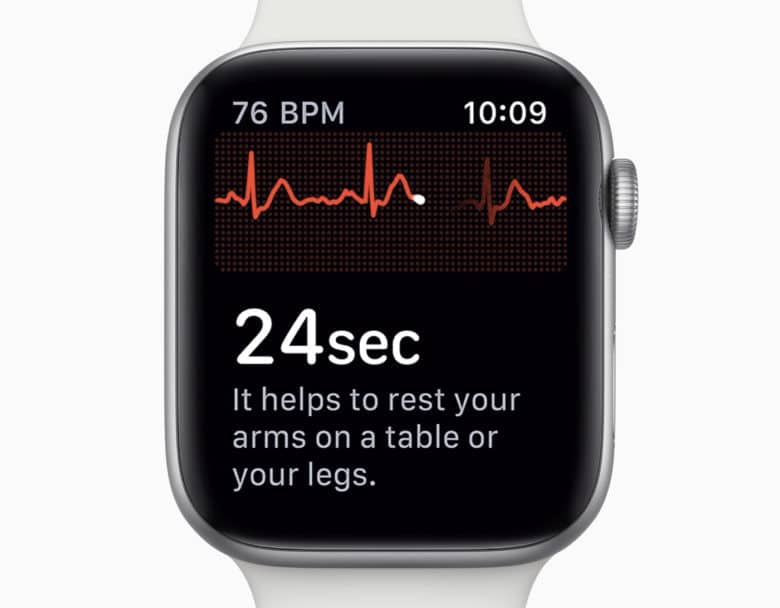
At the unveiling earlier this month, the feature Apple drew a lot of attention to was the electrocardiogram (ECG) feature that specifically measure the electrical signature of your heartbeat. This information is then classified as a heart rhythm which can be shared with your doctor. The feature isn’t live yet but is immediately limited to the United States this year. The FDA have already cleared this functionality so it can be activated as soon as it is available.
In the UK devices with ECG features have to be cleared by the Medicines and Healthcare products Regulatory Agency (MHRA) and they have a lengthier process beginning with examining Apple’s documentation on the ECG and performing a full audit of the quality assurance system.
Before they arrive at this stage though, they require Apple to perform a new clinical investigation to judge the effectiveness of the ECG on the Apple Watch but this is an issue because Apple won’t be able to share the data from studies it has already completed because the MHRA requires companies to notify the regulator in advance of a study.
Have a heart
Once a study is submitted then the MHRA have up to 60 days to approve it, which might go on longer if they have any further inquiries. Once approved then the study can begin. An MHRA spokesperson said: “You may need to carry out a clinical investigation as part of the process to obtain a CE marking for your medical device. You must inform MHRA if you are planning to do this at least 60 days before starting your investigation [providing] some basic details about the investigational device, the intended population, the type of study, and estimated application date.”
Potentially this could mean the process could drag on for years but Apple can find ways to lobby and expediate the process. While the UK remains a part of the European Union, Apple could receive approval from the broader regulatory body and is currently above the MHRA.
In the US, while Apple has FDA clearance for the ECG feature it does not have FDA approval and they specifically do not recommend that the feature is used by those under 22 years of age or those who have already been diagnosed with atrial fibrillation.
They remind users that the app is not designed to replace traditional diagnosis or treatment methods and is intended for informational use only. Apple is also working with Health Canada to bring ECG functionality to the Canadian market but no timeline has been specified as of yet.